Cytochrome bd Ubiquinol Oxidase
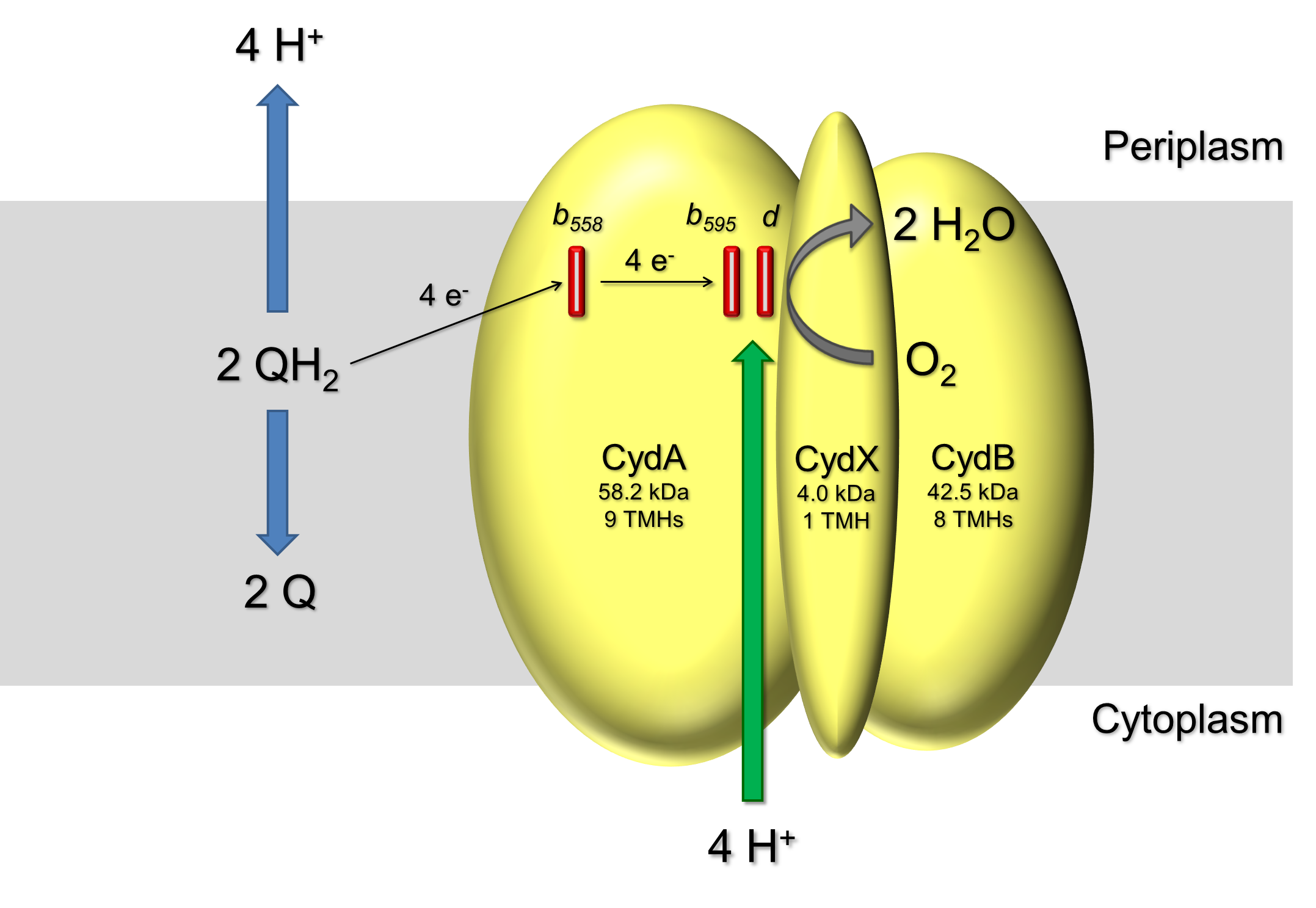
The cytochrome bd ubiquinol oxidase is one of the terminal oxidases present in many prokaryotic respiratory chains. This heme-only oxidase catalyzes the oxidation of ubiquinol to ubiquinone while reducing molecular oxygen to water. This reaction is coupled to the vectorial transfer of 1 H+/e- across the membrane, contributing to the proton motive force essential for energy consuming processes. It is the last enzyme of the respiratory chain without known molecular structure.
Due to its exceptionally high affinity to molecular oxygen, the oxidase is used by bacteria to survive in oxygen-poor environments. On the other hand, it can serve as an oxygen scavenger in order to protect oxygen sensitive enzymes from degradation. Since many human pathogens (tuberculosis, meningitis, pneumonia, ...) rely on these properties, the presence of the bd oxidase is essential for their virulence. This makes the cytochrome bd ubiquinol oxidase a very attractive drug target.
The oxidase can be purified by chromatographic steps and all cofactors can be characterized by UV/vis Redox difference spectroscopy. Our group recently showed, that CydX is a third subunit to this terminal oxidase and is essential for the stability of the b595-d di-heme oxygen reduction site. CydX consists of only 37 amino acids building up a single transmembrane helix and appears to cover the active site.
References
Borisov, V.B., Gennis, R.B., Hemp, J., Verkhovsky, M.I. (2011) The cytochrome bd respiratory oxygen reductases. Biochim. Biophys. Acta 1807, 1398-1413.
Hoeser, J., Hong, S., Gehmann, G., Gennis, R.B., Friedrich, T. (2014) Subunit CydX of Escherichia coli cytochrome bd ubiquinol oxidase is essential for assembly and stability of the di-heme active site. FEBS Lett. 588, 1537-41.